
It was 28 years ago when I began teaching in the Engineering Technology Department at Mohawk Valley Community College in Utica, NY. During that time I’ve taught everything from drafting to engineering dynamics to - you guessed it - hydronic heating.
On December 31, 2008, I retired from full-time teaching at MVCC. Notice the word “retired” begins with a little r. That reminds me I can’t put my feet up on the front porch quite yet. Instead I’ll be working full time through my own company, Appropriate Designs. I hope to use the coming years to push the envelope on design and application of hydronic heating systems a bit further.
An Interesting Visit
Last December I was sitting in my college office contemplating how I would ever clean up what had been accumulating since Ronald Reagan took office.There was a knock at the door.
“Hi, I’m John, and I teach psychology courses here at MVCC. Would you mind if I ask you a really simple and stupid question?” I smiled and welcomed John to sit down among the semi-organized chaos in my cubicle-with-a-door office.
John started talking. “I’m adding onto my house. My plumber said that my water heater produces so much extra heat that I can just run the water from it through tubing in the floor and heat the addition rather than waste the heat. It seems like I would still have to pay for that heat, but am I missing something?”
Although John’s expertise was in psychology, he obviously understood the first law of thermodynamics. Water heaters don’t produce “extra heat” without burning fuel.
I explained that water heaters have a niche in the hydronic heating market. They’re acceptable in situations where a small addition needs heat, but the remainder of the building has something other than hydronic heating.
John followed up with another question, just to be sure. “But even in those cases the owner has to pay for the heat, right?”
That’s right, when it comes to a fuel-burning heat source there’s no such thing as surplus heat.
The Shrink Gets It Right!
We went on to discuss how a boiler is specifically designed to supply space heating whereas a domestic water heater is not. The efficiency of a modern boiler in converting fuel to heated water is significantly better than that of a standard water heater.Even in cases where a water heater did serve as the heat source it’s important to separate the potable water it contains from the water circulating through the space heating distribution system. A stainless steel heat exchanger along with a bronze or stainless circulator will be needed. The space heating distribution system will also need its own circulator along with an expansion tank, air separator, fill/purging valves, and pressure relief valve. By the time all this additional hardware is purchased and installed, the “savings” associated with using the water heater starts to evaporate pretty quickly. Depending on the thermal efficiency and life expectancy of the water heater in this application, the net cost might even be higher than that associated with using a boiler in the first place.
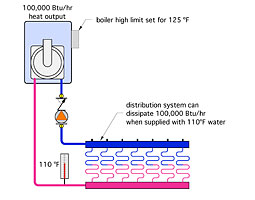
Figure 1.
Nature Likes A Balance
After our conversation, the psychology professor left with a smile on his face. I’m sure it came from being reassured that “extra Btus” aren’t just floating around in the air - or inside a water heater - waiting to be discovered and put to use. Score one for the shrink. His knowledge of basic science helped him sort through a situation that seemed too good to be true.Knowledge of basic science does that. It helps you decide if your next flash of technical inspiration is really a possibility, or only something that could be accomplished on a movie set using special effects.
Time and again I come across questions and design sketches that could have benefited from a bit of scientific introspective, especially before thousands of dollars’ worth of hardware demonstrates that physics trumps inspiration every time.
To that end I recommend you apply a few of the following basics to every hydronic system you design for or review with a customer.
1.Water, like electricity, favors the path of lower resistance.
2.All that water “cares about” when pushed out of a circulator is getting back to the other side of that circulator. It “doesn’t care” if the proper amount of heat goes along for the ride, or gets off at the right location(s).
3.Even though we rely on the circulator to create flow in modern hydronic systems, “gravity flow” is still possible. It can and will show up whenever there is a source of heated water below an unblocked flow path above. You might not spot that path while drawing or installing the system, but be assured nature will find it and exploit it to dump heat where it’s probably not needed.
4.Energy, like every penny of cash flow in a business, must be accounted for. I’m sure you’ve all heard that energy cannot be created or destroyed, only changed in form. This implies that the energy flowing into any device, in any form, cannot simply vanish. It has to go somewhere, and therefore the following is always true for any device operating at a steady state condition: Energy in = energy out.
Temperature is simply the “evidence” of energy flow. If the temperature of an object is increasing, that object is temporarily absorbing energy faster than it’s releasing it. You can see this on the temperature dial of most boilers after they first turn on.
If the temperature of an object is decreasing, it’s releasing energy faster than it’s taking on energy. Watch the water temperature indicator on a boiler after a large cold zone circuit has just turned on. It will drop as heat is temporarily removed from the water faster than the burner can replace it.
When the temperature of an object is steady, the rate of energy flow into and out of that device is equal.
The last principle can be applied to the system as a whole. I like to call this “thermal equilibrium.” It means that every hydronic system seeks to operate at a supply water temperature (to the distribution system) where the rate of heat generation by the heat source is exactly equal to the rate of heat dissipation by the distribution system.
This water temperature isn’t necessarily going to make the system work as expected. It might not even let it run at what we consider safe operating conditions. The water and the energy it carries don’t care about those things. They only care that a balance is achieved between incoming and outgoing heat.
To illustrate this point, consider a system in which a boiler, rated at 50,000 Btu/hr output, is connected in a simple series circuit to 25 feet of finned-tube baseboard. Remove all the safety devices. Install a circulator. Fill it up with water, and turn it on. What’s going to happen?
In short, the system will immediately begin searching for thermal equilibrium. The water temperature will quickly rise, because at 100 degrees F, or 150 degrees F, or even 200 degrees F water temperature, the baseboard cannot release the 50,000 Btu/hr the boiler is injecting into the water.
Based on a few calculations that I won’t bore you with, I determined that if the average water temperature in the baseboard got up to 397 degrees F, that 25 feet of baseboard could dissipate the full 50,000 Btu/hr output.
Impossible, you say. Not necessarily. If the boiler and other components could handle a pressure of say 200 psi this system could - and would - achieve thermal equilibrium. Would this be safe? Not on my watch. Would it be what the system “wants” to do? Absolutely.
Another example of how thermal equilibrium might show up is shown inFigure 1.
In this case, a boiler, rated at 100,000 Btu/hr heat output, has been connected directly to a floor heating system. That radiant floor can dissipate 100,000 Btu/hr into its building when supplied with 110 degrees F water. However, the installer doesn’t know this. Instead he thinks the water temperature needs to be 125 degrees F, and sets the high limit controller on the boiler for this temperature.
When the system is turned on, the boiler fires at full rate, and eventually the water temperature leaving it stabilizes to 110 degrees F. The installer becomes frustrated because the boiler limit controller is set for 125 degrees F, and it’s just not getting there. He can’t figure out what’s wrong with the boiler.
Actually, there’s nothing wrong. This system simply settled into its thermal equilibrium at a supply temperature of 110 degrees F, and that’s all there is to it. There’s no need for the water to climb to higher temperatures to do its job of moving 100,000 Btu/hr from the boiler to the load. The water doesn’t care about the limit control setting on the boiler, it only cares about balancing heat supply with heat dissipation.
Our task in laying out systems is to recognize that:
1.Every system seeks this thermal balance, and assuming controls or safety devices don’t interfere, will eventually attain this balance - unless, of course, one of the piping components explodes on the way there.
2.In light of #1, we must size components so that thermal equilibrium occurs at safe operating temperatures, and preferably at temperatures that maximize the efficiency of the heat source.
So, just like my colleague the psychology professor, stand back and see if the systems you design, or review with customers, comply with basic scientific principles. Remember, thermodynamics isn’t just a good idea - it’s the LAW!
